UNIQUE mode of action of ustekinumab:
IL12/IL23-receptor binding inhibition
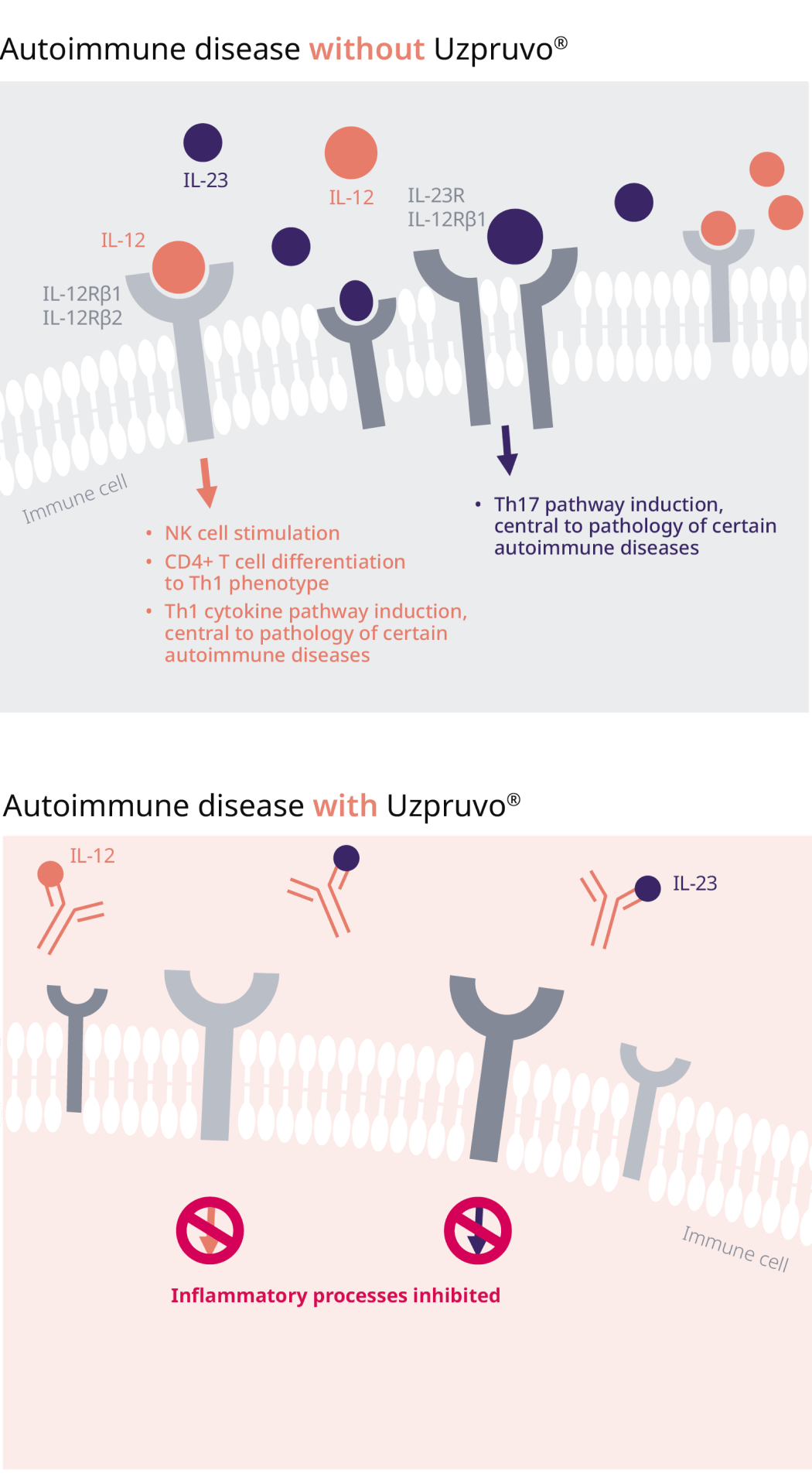
Ustekinumab is a fully human IgG1κ monoclonal antibody that binds with specificity to the shared p40 protein subunit of pro-inflammatory cytokines IL-12 and IL-23 to prevent them from binding to their receptors, expressed on the surface of immune cells, therefore inhibiting inflammatory processes early1
UZPRUVO® IN GASTROENTEROLOGY:
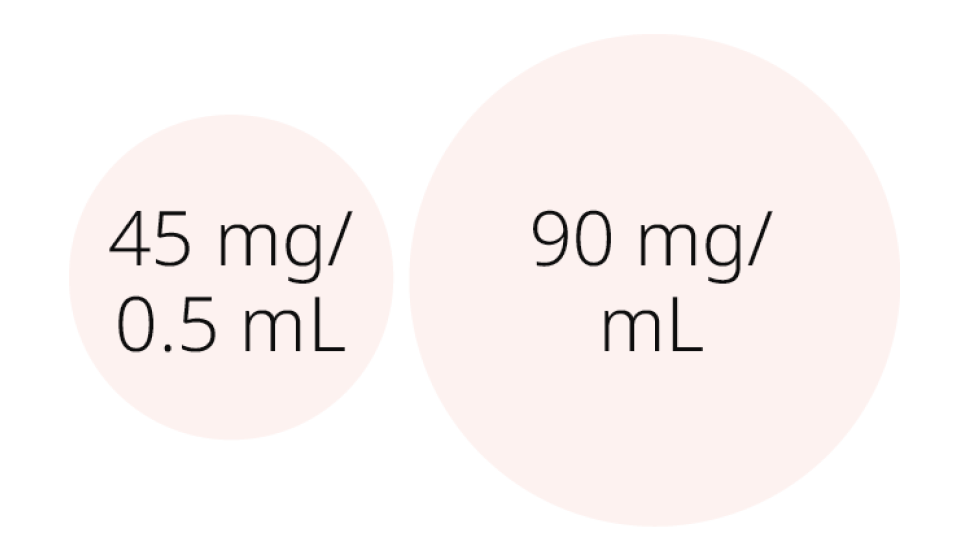
DOSE STRENGTH OPTIONS1
Subcutaneous dose strengths for maintenance treatment
Maintenance:
SC Injection*
Presentation:
Pre-filled Syringe
SC, subcutaneous
*The approved ustekinumab treatment regimen for patients with Crohn's disease is initiation with a single intravenous dose based on body weight. Uzpruvo® is available in pre-filled syringes for subcutaneous use. After the first intravenous infusion induction dose with an alternative ustekinumab product, patients can receive subcutaneous maintenance doses with Uzpruvo®.1
Dosing Scheme1
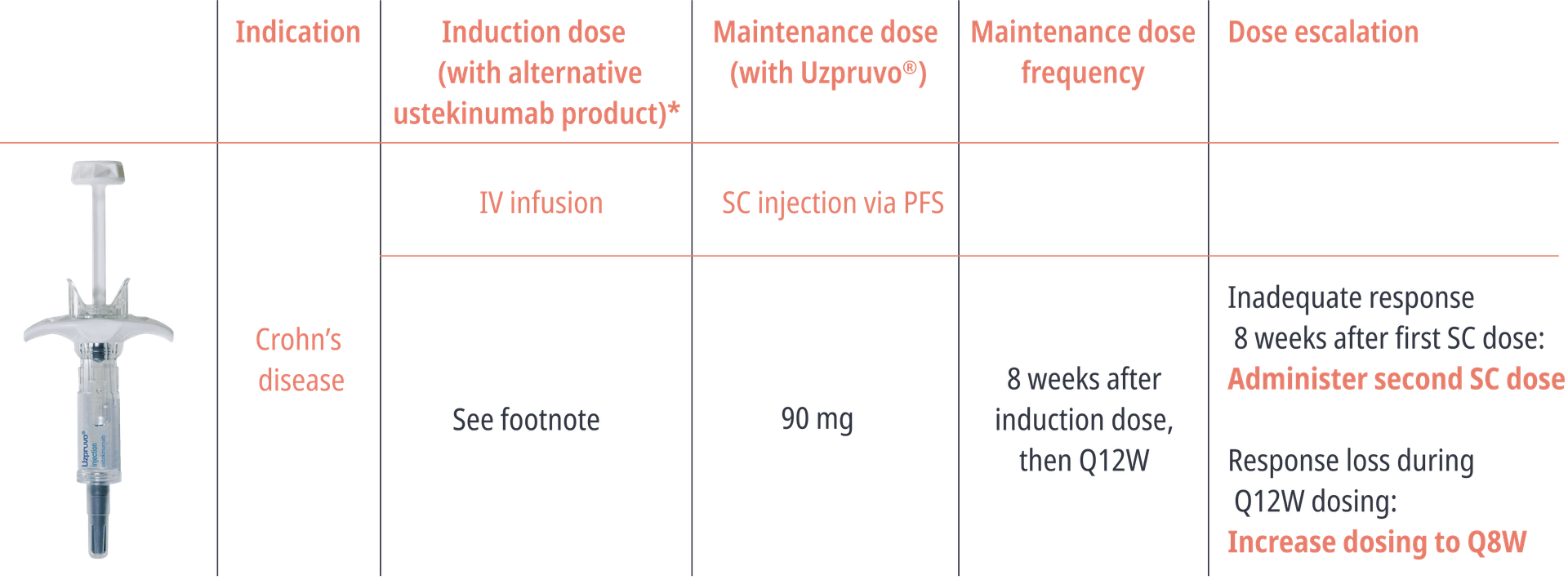
PFS, pre-filled syringe; Q8W, every 8 weeks; Q12W, every 12 weeks; SC, subcutaneous *The approved ustekinumab treatment regimen for patients with Crohn's disease is initiation with a single intravenous dose based on body weight. Uzpruvo® is available in pre-filled syringes for subcutaneous use. After the first intravenous infusion induction dose with an alternative ustekinumab product, patients can receive subcutaneous maintenance doses with Uzpruvo®.1
Comparatively Fewer Treatments:
Gastroenterology Treatment Regimes1-3
Example of total doses in the first year of treatment (52 weeks) for adult patients with Crohn's disease
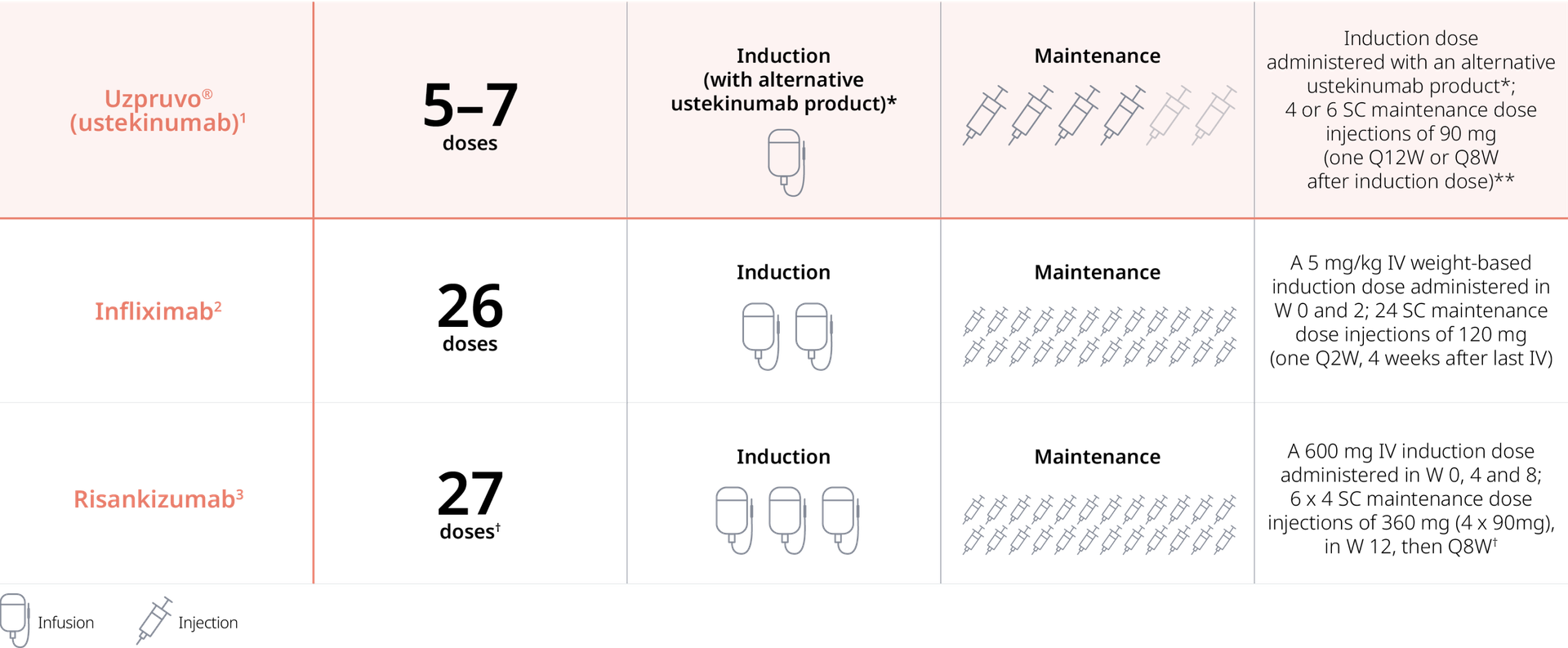
IV, intravenous; PFS, pre-filled syringe; Q2W, every 2 weeks; Q8W, every 8 weeks; Q12W, every 12 weeks; SC, subcutaneous; W, week(s)
*The approved ustekinumab treatment regimen for patients with Crohn's disease is initiation with a single intravenous dose based on body weight. Uzpruvo®1 is available in pre-filled syringes for subcutaneous use. After the first intravenous infusion induction dose with an alternative ustekinumab product, patients can receive subcutaneous maintenance doses with Uzpruvo®1; **Patients who lose response on dosing Q12W may benefit from an increase in dosing frequency to Q8W1; †Number of maintenance injections given with a PFS. The number of injections with an on-body-catridge is 63
1. Uzpruvo® SmPC 2. Remsima® SmPC (Jan. 2025); 3. Skyrizi® SmPC (Jan. 2025).
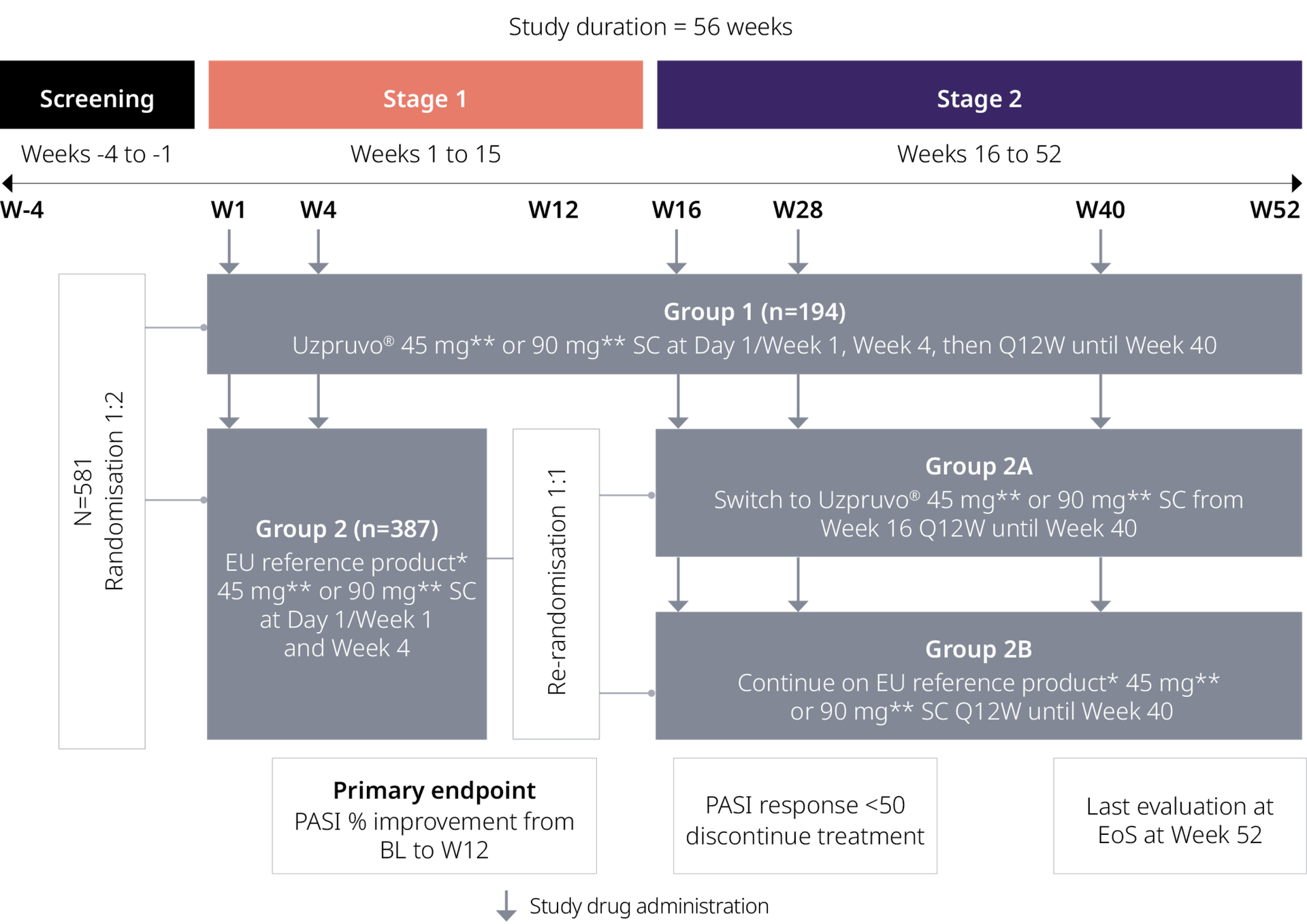
Adapted from Feldman SR et al. 20231
BL, baseline; BSA, body surface area; DLQI, Dermatology life quality index; EoS, end of study; PASI, Psoriasis Area and Severity Index; Q12W, every 12 weeks; SC, subcutaneous; sPGA, statistic physician's global assessment
*Stelara®; **≤100 kg body weight: 45 mg, >100 kg body weight: 90 mg
1. Feldman SR et al. Expert Opin Biol Ther. 2023;23(3):253-60. DOI: 10.1080/14712598.2023.2235263.
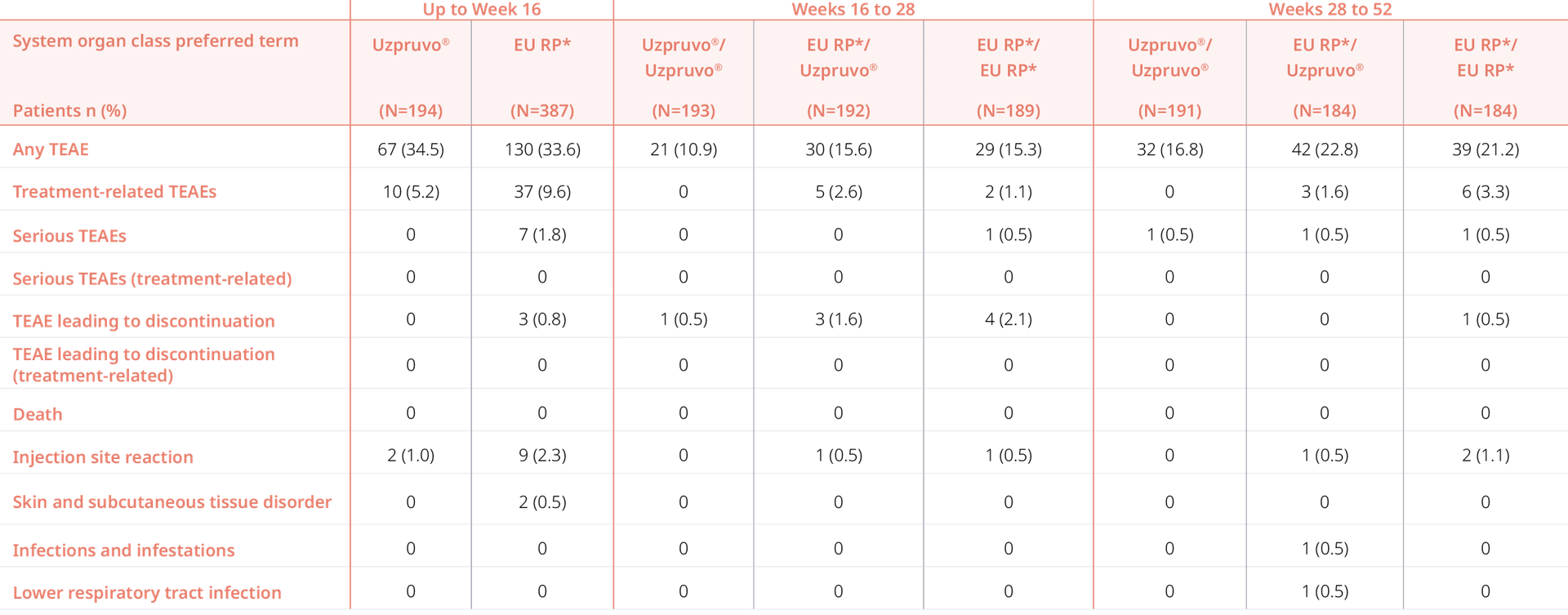
Adapted from Feldman SR et al. 20231
AE, adverse event; RP, reference product; TEAE, treatment-emergent adverse event
*Stelara® 1. Feldman SR et al. Expert Opin Biol Ther. 2023;23(3):253-60. DOI: 10.1080/14712598.2023.2235263.